
How does a helpful substance like cholesterol turn deadly?
Israeli scientists show how this basic component of body cells can become ‘bad’ and cause hardening of the arteries.
You probably know that LDL cholesterol is “bad” and that too much of it in your blood puts you at risk of atherosclerosis, hardening of the arteries.
The job of cholesterol is to provide elasticity to the fatty substance that makes up cell membranes. LDL (low-density lipoprotein) cholesterol acts as “packaging” to help cholesterol travel through the blood, and can even clear away molecular cholesterol that settles on blood vessel walls.
The trouble seems to begin when crystals of cholesterol form. These are the material of the plaques that form in lesions, causing irreversible damage that leads to atherosclerosis and eventually to the heart disease and stroke that are the leading killers in the Western world.
In their research findings published in the Proceedings of the National Academy of Sciences (PNAS), the scientists showed that these harmful cholesterol crystals form in two different ways in immune cells that unwittingly aid in the progression of the disease.
A pathological chain of events
When levels of LDL in the blood are too high, some of it is deposited on the blood vessel walls. There, the cholesterol undergoes oxidation, making these molecules toxic to the cells lining the blood vessels.
When the immune system recognizes an arterial lesion caused by this oxidation process, it sends “Pacman”-like cells called macrophages to “eat up” the harmful material. But macrophages that overeat– meaning they have too much unwanted stuff to clean up — inflate, turn foamy and die.
The dead foam cells and escaped fatty molecules buildup at the site of the original arterial lesion, worsening the inflammation and leading to the formation of cholesterol crystals.
“The buildup of cholesterol crystals is a major step in a pathological chain of events that includes cell death, inflammation and the onset of arterial disease. We find that the crystals can form very early on in this chain,” said Prof. Lia Addadi of the Weizmann Institute’s Structural Biology Department.
The research was led by Neta Varsano, a post-doc in Addadi’s lab.
“Crystals are observed outside of the macrophages but also inside them, and it has been difficult to understand precisely when these begin to form,” Varsano said.
She and the research team collaborated with the lab of Prof. Leslie Leiserowitz of Weizmann’s Materials and Interfaces Department to devise a method that would enable them to trace the process.
To observe the crystallization process, the team grew macrophage cells and added LDL cholesterol to them. They then observed them using a combination of methods including high-resolution fluorescent microscopy and a type of cryogenic X-ray imaging.
As a result, the team was able to look atuniquely detailed, three-dimensional images of the crystals and follow their early growth.
The earliest crystal structures they detected were thin, flat rhomboid-shape platelets in the cells’ membranes. Then they discovered a larger type of crystal, elongated and needle-shaped, inside the cells. These appeared to act like needles that prick the cell membrane and rupture it.
Addadi said that these larger crystals were a surprise. They don’t show up in the standard test-tube studies of cholesterol crystals.
Together with Dr. Nadav Elad of the Institute’s Chemical Research Support, Addadi’s lab characterized the crystalline structure of each formation. They noted that the elongated crystals usually were organized around a hollow or cylindrical tube, but were sometimes helical in shape, similar to crystals related to gallstone formation.
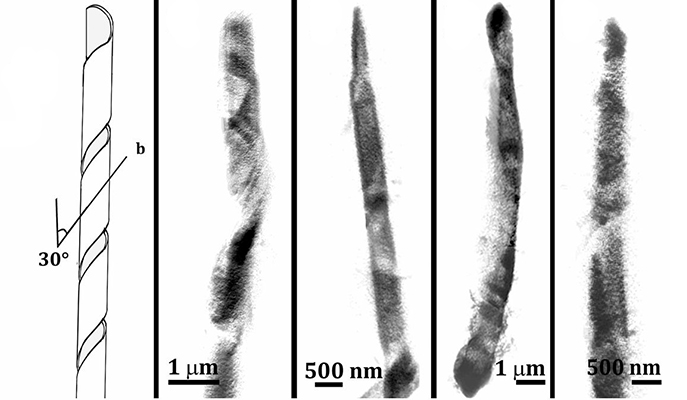
Varsano said more work is needed to completely solve the riddle of how cholesterol transforms into killer crystals. The research team is now comparing samples from different people to see if the crystals form in the same way in everyone or if there are variations.
Addadi has dedicated her career to investigating how crystals work in living creatures. She was recently elected a member of the American Academy of Sciences, and this study on cholesterol crystals was her inaugural paper to its journal.
Also participating in this research were Tali Dadosh and Iddo Pinkas of the Weizmann’s Chemical Research Support; Fabio Beghi of the University of Milan; Eva Pereiro and Ana Perez-Berna of the ALBA synchrotron in Spain; and Prof. Howard Kruth of the Experimental Atherosclerosis Section, National Heart, Lung, and Blood Institute, US National Institutes of Health and members of his group.