Experimental Israeli-made drug Allocetra, developed at Jerusalem’s Hadassah hospital, is being used to treat the so-called cytokine storm, whereby immune system attacks body’s own organs. A second Israeli drug is making waves, too. PM Netanyahu: “If this succeeds, it’s huge.”
The experimental Israeli-made drug Allocetra, used to treat serious and critical COVID-19 cases, has completed Phase 2 trials successfully.
Of 20 seriously ill patients treated with the drug so far, 90% recovered, Israel’s Channel 13 news reported Tuesday.
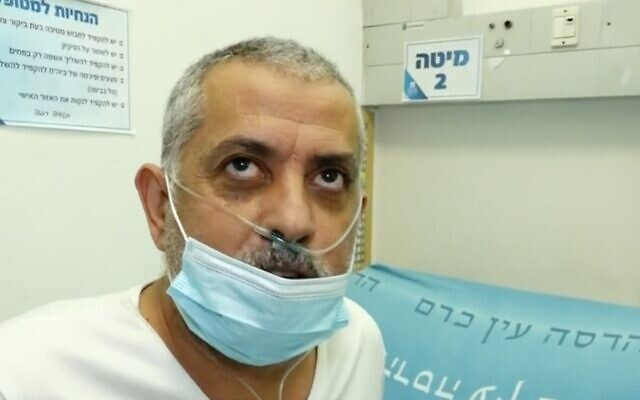
“I couldn’t breathe, I could barely speak. [I was in] very very serious condition,” Tayeb said. “I went through an experience you can’t put into words.”
Within two hours of receiving the drug, he said, he felt a change. “They gave me the drug. Suddenly after two hours I started feeling something strange in my body. I stopped coughing, my breathing started to come back, I was feeling better. I stopped sweating. I couldn’t believe it. I was afraid to tell people I was okay, I was so excited.”
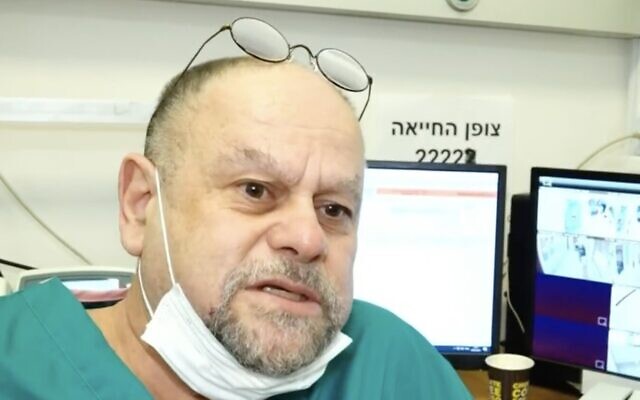
Prof. Dror Mevorach, head of one of Hadassah’s coronavirus wards and chief scientific and medical officer at Enlivex, who developed the treatment, told Channel 13: “It is useful for serious and critical patients because it can prevent the need to ventilate them, and that’s the major goal. Because the moment you go into ventilation, the entire situation changes, complications rise, and it’s more difficult to treat.”
The drug is now entering Phase 3 trials and will be given to over 100 people.
“Two days ago I couldn’t stand on my legs,” Tayeb said as he left the hospital. “Look at me now, going home.”
A second Israeli COVID-19 drug, developed at a Tel Aviv hospital, is also making waves. Tel Aviv’s Ichilov Medical Center claimed a “huge breakthrough” on Friday, saying that Prof. Nadir Arber’s EXO-CD24 inhaled medicine had been administered to 30 patients whose conditions were moderate or worse, and all 30 recovered — 29 of them within three to five days.
(Amos Ben-Gershom/Israeli Government Press Office)
Arber told The Times of Israel on Tuesday that, with the Phase 1 trial just completed, he has applied to the Health Ministry to start a Phase 2 trial for the EXO-CD24 inhaled drug. This will give a more reliable picture of efficacy, as Phase 1 is small, largely concerned with checking safety, and lacking a placebo group.
On Monday, Prime Minister Benjamin Netanyahu invited Arber to his office and asked him about the “miracle drug.” During the briefing, Netanyahu said: “If this succeeds, it will be huge, simply huge. This is of global significance.”
(Times of Israel).
